SnakeBite911 FR
For Emergency First Responders
Venomous snakebites are often seasonal and infrequent occurrences that need careful and immediate management.
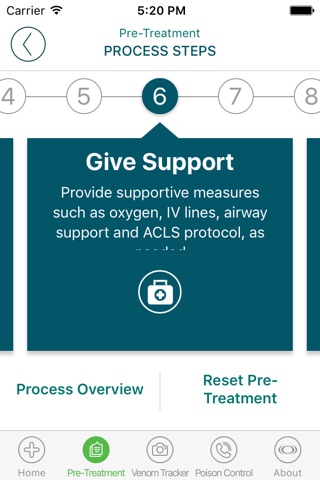
SnakeBite911 FR, for Emergency First Responders, is a guide for medically-trained emergency personnel that walks through a checklist of best practice actions recommended to be carried out in a snakebite emergency from venomous pit viper snakes, including rattlesnakes, copperheads, and cottonmouths. These actions aim to help the bite victim be appropriately managed from pick-up by the First Responders through to delivery at a snakebite-treatment proficient Emergency Department.
This App provides First Responders with useful information so they can act quickly and with confidence in a snakebite emergency where time is tissue!
Features
• Actions to avoid in a snakebite emergency
• Best practice pre-treatment steps to carry out from victim pick-up
• Time-stamped Venom Tracker tool to help capture envenomation progression at bite location
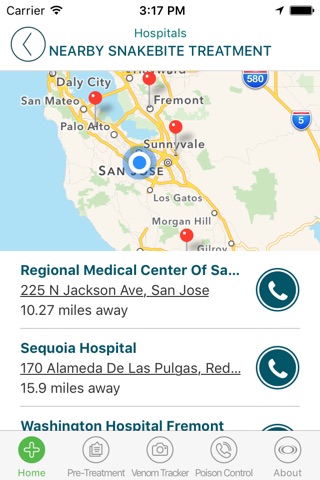
• Locate nearby hospitals that may stock CroFab® to treat venomous North American pit viper (crotalid) bites
• Quick dial to the Poison Control Center, for expert advice on managing venomous snakebites
• Quick access to information about CroFab®
The SnakeBite911 FR app comes from the manufacturers of CroFab® Crotalidae Polyvalent Immune Fab (Ovine), an antivenin product clinically proven to halt progression of envenomation from venomous North American pit viper (crotalid) bites. Find out more at www.CroFab.com, which includes full Prescribing Information (http://www.crofab.com/documents/CroFab-Prescribing_Information.pdf) and Important Safety Information (http://www.crofab.com/#section-isi).
This App is not intended for use by the general public. If you are a patient or a caregiver for a patient, please contact your healthcare professional for advice.
Selected Safety Information about CroFab®
In clinical trials, recurrent coagulopathy (the return of a coagulation abnormality after it has been successfully treated with antivenin), characterized by decreased fibrinogen, decreased platelets, and elevated prothrombin time, occurred in approximately half of the patients studied. Recurrent coagulopathy may persist for 1 to 2 weeks or more. Patients who experience coagulopathy due to snakebite should be monitored for recurrent coagulopathy for up to 1 week or longer.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.FDA.gov/medwatch or call 1-800-FDA-1088.
ZINC ref: NA-CRF-2016-0138